![Figure 1: SEM micrographs of polished section of alite sample exposed to portlandite solution for 1 hour from Juilland and Gallucci [8] Grain boundaries becomes visible as they are preferential sites for dissolution etching.](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_1.5b242d063f54f.png?auto=format%2Ccompress&fit=crop&h=288&q=70&w=512)
Modern concrete is strongly dependent on the reliable performance of admixtures. The pressures of minimizing cost and environmental impact, accelerating construction schedules, reducing concrete placement labor, and improving concrete durability have combined to make admixture use a virtual requirements. Frequently, several admixture technologies may be combined to ensure that performance specifications are met. Furthermore, the desire to reduce the environmental impact of cement clinker manufacture has also led to the use of multiple secondary cementitious materials (SCMs) having widely differing compositions, reactivities, surface areas, absorptive capacities and other factors. Admixtures help maintain the desired performance characteristics of the SCM concrete systems, and thus substantially reduce the environmental footprint due to lower clinker factors. As future pressure on sustainability will continue to require increasingly complex combinations of cementitious materials, likely with higher aluminum content and higher admixture use [1,2] it is important to develop a better understanding of how to predict and manage their collective behavior. In a previous paper by Cheung et al [3], sulfate optimization and admixture-cement interactions were extensively reviewed. In this work, the authors present an updated literature review on a new fundamental and mechanistic understanding of how cement-admixtures interact, and how advances in the industry have contributed towards meeting several technological challenges. Additionally, real-world examples on the use of (1) new laboratory and field-deployable calorimetry techniques and (2) cutting-edge in-transit concrete management systems that enable more sustainable concrete production will be provided. Despite these notable improvements, this paper will also identify technical challenges that more sustainable concrete systems will create for admixture usage.
Current Understanding of Cement Hydration and Cement-SCM-Admixture Interactions
Today, many concrete applications require high strength development in as little as a few hours. Both the cement and concrete industries seek refined manufacturing strategies to meet such ambitious targets. Cement hydration is an ongoing dissolution-precipitation process taking place at the cement grain/solution interfaces. Although current exploratory methods focus on changes occurring at the interface, additional advanced techniques with even higher resolution and specifications would enable researchers to investigate cement hydration events occurring at the nano and even atomistic scale. Understanding the interface reactions during cement hydration is critical to the development of high-quality cementitious systems. This section reviews the recent progress made in the field, and suggests areas where these new findings may pave the way to optimal hydration of sustainable concrete.
Cement Hydration
The Silicate Phase
To date, a majority of the research conducted on cement hydration has focused on the hydration of its most prevalent phase, tricalcium silicate (C3S). This particular phase represents from 40 wt-% to 70 wt-% of an average cement particle, and the kinetics of hydration are very similar to those of Ordinary Portland Cement (OPC).
Scrivener et al. [4], summarized the current understanding of cement hydration and pointed out that dissolution of C3S over the initial wetting and induction stages plays a key role in determining the nature of cement hydration. In practice, this period corresponds to the transportation and placement process of concrete at the work site. The ability to tune this critical period of hydration is essential to the production of good quality concrete.
In 2010, Juilland et al. [5] suggested that the induction period of cement hydration is controlled by the dissolution of C3S, and not limited by a hydrate barrier, as previously proposed by Gartner [6] Subsequently, Nicoleau et al. [7] and Juilland and Gallucci [8] further explored the dissolution of C3S and researchers concluded that the dissolution rate of C3S is not linear with time. Their results showed that C3S dissolution rate is strongly dependent on the chemical pore solution environment. The rate of dissolution changes with the concentration of species: it slows down significantly when the calcium concentration reaches a threshold near the saturation point with respect to portlandite (CH). In geochemistry, the switch from a fast to slow dissolution regime is ascribed to a change in the mechanism of dissolution that is dependent on the undersaturation of the solution. For a high undersaturation level, two-dimensional vacancy islands can nucleate on surfaces without initiation by crystal defects. At a medium undersaturation level, the activation energy barrier increases and dissolution can only occur at dislocation sites or other defect sites that possess an excess of surface energy. As undersaturation decreases further, etch pits can no longer form due to higher energy requirements, and the dissolution rate subsides. In general, dissolution occurs at low undersaturation by step retreat, typically at the site of surface imperfections.
![Figure 2: Topological map of a polished alite sample exposed for 2 hours to saturated portlandite solution from Juilland and Gallucci [8]](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_2.5b242d0550278.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 2: Topological map of a polished alite sample exposed for 2 hours to saturated portlandite solution from Juilland and Gallucci [8]
Figure 2: Topological map of a polished alite sample exposed for 2 hours to saturated portlandite solution from Juilland and Gallucci [8]
Pictures of alite samples exposed to saturated portlandite solution from Juilland and Gallucci reveal preferential etching at the edges of the grain boundaries and at surfaces damaged by the polishing [8]. The dark lines shown in Figure 1 illustrated the edges of the crystallographic grains due to deep pitting of a polycrystalline alite sample. Using Vertical Scanning Interferometry on similar alite samples, they also observed that each grain has a different grey level, indicating variation in the etching depth (Figure 2). This finding suggests that dissolution is heterogeneous, and each grain is dissolving at a different rate, which most likely depends on the crystallographic plane.
![Figure 3: Cryo SEM micrograph of a cement grain sample hydrated for 6 hours with C-S-H seeds from Nicoleau and Bertolim [9]. Etch pits are indicated by white arrows and localized on one plane only.](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_3.5b242d0498e2c.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 3: Cryo SEM micrograph of a cement grain sample hydrated for 6 hours with C-S-H seeds from Nicoleau and Bertolim [9]. Etch pits are indicated by white arrows and localized on one plane only.
Figure 3: Cryo SEM micrograph of a cement grain sample hydrated for 6 hours with C-S-H seeds from Nicoleau and Bertolim [9]. Etch pits are indicated by white arrows and localized on one plane only.
![Figure 4: Cryo SEM micrographs on a section of cement grain hydrated for 20 hours from Nicoleau and Bertolim [9]. The etch pitting orientation is indicated by red lines. Pitting direction appears to be dependent on the crystal planes of the alite.](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_4.5b242d0626914.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 4: Cryo SEM micrographs on a section of cement grain hydrated for 20 hours from Nicoleau and Bertolim [9]. The etch pitting orientation is indicated by red lines. Pitting direction appears to be dependent on the crystal planes of the alite.
Figure 4: Cryo SEM micrographs on a section of cement grain hydrated for 20 hours from Nicoleau and Bertolim [9]. The etch pitting orientation is indicated by red lines. Pitting direction appears to be dependent on the crystal planes of the alite.
Experiments from Bazzoni et al. demonstrate that by producing or removing crystal defects, the reactivity of C3S and therefore the induction period kinetics can be controlled [10]. In practice, crystal defects can be created by either incorporation of impurities, quenching treatment, and/or by mechanical grinding techniques. The dependence of hydration rate on the reactivity of defects, representing a low percentage of the total silicate surface, may help explain why some admixtures can strongly influence dissolution rates at doses far below those required to provide complete surface coverage.
The grain surface area increases during the acceleratory period, and this can be attributed to the presence of etch pits on the C3S surface and subsequent growth of C-S-H hydrates. The precipitation and growth of C-S-H is considered to be the dominant kinetic mechanism that drives the acceleration period. Scrivener et al.[4] has reported supporting evidence of this theory which suggests that a reduction in the rate of acceleration is caused by a lack of available surface to support C-S-H growth.
However, more recent research findings propose alternate mechanisms. Thomas [13], for example, demonstrated that the activation energy of C3S and cement hydration over the first 24 hours is constant. This reported apparent activation energy is dependent on the rate limiting mechanism. A constant activation energy implies a single mechanism controlling the hydration; this result contradicts the theory posited by Nicoleau and Nonat [14], and Juilland et al. [5] stating there is a change in mechanism when the induction period transitions to the acceleration stage. Scrivener et al. suggests one possible reason for the monotonic activation energy observed could be that the technique used to capture the overall activation energy of hydration does not distinguish the different rates of mechanisms involved in the hydration process [4]. Another explanation proposed by Nicoleau and Nonat suggests that this can be explained by accounting for the C3S dissolution area over the hydration process, instead of simply considering the initial nucleation surface area of C3S, because the dissolution surface area continues to increase as etch pits form [14]. Dissolution increases with the surface area of etch pit walls. In the case of the deep pitting, the ratio of surface area within the pit wall to the initial area of the surface, before pitting, will be very large and grow rapidly. At a specific moment, etch pits start to coalesce. This reduces the surface area, thus slowing down the dissolution rate. As a result, the available surface area for dissolution of C3S could be the sole governing mechanism of C3S hydration and, to some extent, of cement hydration. This theory is consistent with Thomas’s findings regarding constant activation energy over the first day of hydration.
Regardless, both theories can explain the S-shape curve of the kinetics of hydration obtained by isothermal calorimetry, and both are supported by different mathematical models [9,15–17].
In conclusion, these results highlight the effect of the C3S dissolution on C3S hydration, and more broadly, the impact on cement hydration. Physical characteristics of the surface, as well as the chemical composition of the solution at the C3S/liquid interface, are of great importance. The relative impact of dissolution control versus nucleation and growth control has yet to be fully elucidated.
Fortifying the current understanding of silicate dissolution is a major challenge. One obstacle that needs to be addressed in the future is the development of suitable techniques that enable the investigation of hydration in conditions representative of those in cement paste. Unfortunately, a number of the techniques discussed in this paper were utilized with solution-to-solid ratios higher than those used in general practice, resulting in conclusions that may not accurately reflect reality.
Impact of Aluminum on the Silicate Phase
The impact of aluminum (Al) on the silicate phase has recently been a topic of great interest due to the increased amount of aluminum in low-CO2 cement-based materials such as cements blended with either SCMs or Al-rich cement (e.g. CSA).
Earlier studies investigated the impact of aluminum on C3S dissolution [18], alite hydration [19], micro reactions of alite [20], silica [21] and polyphase cement [22], and in all cases, aluminum negatively impacted C3S hydration. Nevertheless, the explanation for this observation is still under discussion.
![Figure 5: SEM micrographs of amorphous silica exposed to NaOH for dissolution over 90 days from Chappex [21]. a) initial silica surface, b)silica surface treated with 30mM Al and 0.2 M NaOH, c) silica surface with no Al treatment and 0.6M NaOH, d) silica surface treated with 30mM Al and 0.6M NaOH](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_5.5b242d09822f7.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 5: SEM micrographs of amorphous silica exposed to NaOH for dissolution over 90 days from Chappex [21]. a) initial silica surface, b)silica surface treated with 30mM Al and 0.2 M NaOH, c) silica surface with no Al treatment and 0.6M NaOH, d) silica surface treated with 30mM Al and 0.6M NaOH
Figure 5: SEM micrographs of amorphous silica exposed to NaOH for dissolution over 90 days from Chappex [21]. a) initial silica surface, b)silica surface treated with 30mM Al and 0.2 M NaOH, c) silica surface with no Al treatment and 0.6M NaOH, d) silica surface treated with 30mM Al and 0.6M NaOH
Recently, there has been more evidence supportive of the notion that aluminum is interacting with Si on both C3S and C2S surfaces [18]. Since this interaction should preferentially occur at high energy sites, i.e. at crystal defect sites where dissolution takes place, Al may inhibit the C3S dissolution by blocking the C3S reactive sites. Experiments by Chappex and al. [21] revealed that a low number of etch pits are formed on model amorphous silica surfaces when the silica has been exposed to Al solution (Figure 5b and 5d), whereas many etch pits were visible on surfaces where no Al is present (Figure 5c). Complementary XPS analysis of the surface clearly depicts the incorporation of Al into the silica framework, rather than adsorption on the silica surface. The covalent bonding between Al and silicate surfaces was confirmed by NMR and XPS measurements from Nicoleau and Nonat [18]
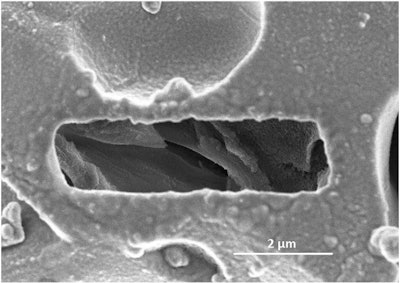
![Figure 6b: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/6b.5b242d4f8b8bb.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 6b: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]
Figure 6b: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]
Further research by Suraneni and Flatt confirmed the negative effect of Al on C3S dissolution. Suraneni used Ga-FIB to form a hole in the C3S sample that was later exposed to different solutions. Figure 6a illustrates the gap after a 4-hour exposure to distilled water. They observed that the gap between walls had increased after hydration compared to the initial dimension of the gap. When a similar gap was exposed to a sodium aluminate solution the wall topography remained intact even after 4 hours of hydration, unlike in pure water where the walls were dissolved (Figure 6b). However, they noticed that the reduction of the dissolution was not as effective at higher pH such as pH 13. The sample exposed to sodium aluminate at pH 13 resulted in walls largely dissolved (Figure 6c), similar to the sample in pure water (Figure 6a). The presence of small nuclei on the base and surface of sample with aluminum suggests that aluminum retards the dissolution mechanism, and not the nucleation and growth of C-S-H, as proposed by Begarin et al. [19]. Begarin et al. suggested that the presence on Al in a synthetic C-A-S-H was the key contributor to the delayed nucleation and growth of the resultant hydration products.
![Figure 6c: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/6c.5b242d50591d1.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 6c: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]
Figure 6c: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]
![Figure 6d: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/6d.5b242d5543609.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 6d: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]
Figure 6d: Images of the gap in C3S sample after 4 hours of hydration exposed to a) pure water, b) 60mM sodium aluminate at pH 12.5: low dissolution, c) 60mM sodium aluminate at pH 13: large dissolution, d) 0.3% superplasticizer: low dissolution compare to pure water from Suraneni and Flatt [20]
These insightful results open new doors for improved understanding of the strong implications of Al in cement hydration. Further work is necessary to identify the role of incorporation or competitive adsorption of Al with other species at the C3S surfaces, especially with the complexity of pH and admixture usage altering the effect. Understanding Al inhibition of C3S dissolution may help to explain the adverse effects of superplasticizers on C3S dissolution. In the work of Suraneni (Figure 6d), C3S samples exposed to superplasticizer show similar results to those exposed to aluminum at pH 12.5 (Figure 6c), suggesting a similar inhibiting effect of C3S dissolution.
Impact of Sulfate on the Silicate Phase
The requirement of sulfate to control the tricalcium aluminate (C3A) reaction has been well established. However, previous work also suggests that sulfate affects the hydration of the silicate phase as well [22].
Beyond the direct effect of gypsum promoting pure C3S hydration shown by Bentur [23], more recent results reveal that sulfate from gypsum, or other sources, positively influences alite hydration. Alite is the impure phase of C3S in cement, and usually contains Al impurities. The reason for this complementary effect can be attributed to the passivation of aluminum by sulfate ions which result in the lower quantities of aluminum in solution. Otherwise, the presence of the aluminum would lead to a reduction in dissolution at the silicate sites.
Direct effects of sulfate on the silicate phase hydration have been shown using atomistic simulation [24], SEM observation [24–26] and dissolution experiments [18]. In the presence of sulfate, CH crystals are elongated, indicating a preferential growth identified by Galmarini et al. [24]. Nucleation might also be affected because formation of the metastable CaSO4 complex may be involved in the change of growth and nucleation of CH. In this case, CH crystals are noted to be smaller and more distributed in the paste with sulfate. This is to some extent consistent with results from Nicoleau et al. on C3S, which reveal the strong interaction of sulfate with Ca at the C3S surface [18]. Elemental Ca at either CH or C-S-H surface may be affected in the same way by sulfate as Ca on C3S surface.
![Figure 7a: SEM micrographs from Mota et al. [25] a: C-S-H showing converging growth in plain alite system b: C-S-H with diverging structure in 5% Gypsum and alite system.](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/7a.5b242d4f16437.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 7a: SEM micrographs from Mota et al. [25] a: C-S-H showing converging growth in plain alite system b: C-S-H with diverging structure in 5% Gypsum and alite system.
Figure 7a: SEM micrographs from Mota et al. [25] a: C-S-H showing converging growth in plain alite system b: C-S-H with diverging structure in 5% Gypsum and alite system.
The conversion to convergent growth of C-S-H, once sulfate depletion has occurred, will be dependent upon the availability of sulfates. Further investigation to provide an improved understanding and prediction of the required sulfate balance in Portland cement is required to establish viable pathways to produce sustainable concrete.
Effect of Sulfate on C3A
For a long time, it has been demonstrated empirically that sulfate enables the control of C3A reactions. However, the mechanism behind this control was not well understood until recently.
Results from Quennoz have demonstrated the relationship of the control of C3A by sulfate to the surface area of C3A [27]. Fine C3A grains reacted faster than coarse C3S grains exposed to the same amount of gypsum, suggesting that sulfate was adsorbing on C3A surface area. Adsorption of SO42- caused by the formation of CaSO4 complex on C3A surface would occur in a similar way to that of a C3S surface [18]. As a result, C3A dissolution is inhibited until the sulfate ions desorb from its surface. This happens in Portland cement when sulfate, in solution, is totally consumed by ettringite formation, leading to the desorption of sulfate from C3A surface in pore solution. More details on research conducted on the mechanism of the C3A inhibition can be found in the review paper by Scrivener et al.[4].
Although the impact of sulfate on C3A can be studied by in situ XRD, it is more common to employ isothermal calorimetry. The end of the inhibition of sulfate on the C3A reaction corresponds to the peak during the deceleration period. It is well known that the depletion of sulfate must happen during the deceleration stage of silicate hydration to maintain good performance and rheology [28]. SCMs in blended cement would also impact the second dissolution of the C3A phase. In blended cement, it is common to have an earlier C3A dissolution. The corresponding shoulder peak has been reported as exacerbated or even doubled by the presence of SCM or mineral filler [29–31] indicating a filler effect in addition to a SCM reaction. However, it is not clear if this effect is caused by a higher water/cement ratio (due by the replacement level of SCM) [22], faster depletion of sulfate, or by more surface area to form ettringite [26]. From the perspective of using more SCM and at higher replacement levels, this question needs to be addressed.
Long Term
Increasing sustainability of cement by using more SCMS usually leads to improvement in durability of concrete. Although kinetics are much slower after one day of curing, many reactions continue to occur that are important for the long-term development of strength and reduction of permeability, including on-going formation and densification of C-S-H, continuous changes in the pore solution environment, and formation of hydrates in confined space. Research on hydration of cement beyond one day has been scant, but recently there has been an increased interest in the longitudinal studies [4]. Admixtures to improve shrinkage, inhibit corrosion, and improve the microstructure of concrete, by reducing water usage, are necessary to manufacture more sustainable concrete. Additional research on understanding how these admixtures work with the new mix designs incorporating more SCMs will be required.
Cement-SCM-Admixture Interactions
Although admixtures were acknowledged as indispensable in making sustainable concrete [1], the technical challenges encountered by the use of higher levels of admixtures, different types of admixtures, and higher levels of SCMs persist. These primary challenges are to avoid deleterious hydration issues such as flash set, set retardation, and slow strength development. In this section, we present a summary on some of the most critical findings about how superplasticizers, retarders and accelerators interact with cement interfaces and show how these interactions impact cement hydration. Additionally, methods to mitigate potential issues are discussed. These findings will also help define future research needed to facilitate development of more advanced admixtures to improve concrete sustainability.
Superplasticizers
Superplasticizers represent the most commonly used type of admixture; for example, 75% of the admixtures used in China are superplasticizers [2]. Water reduction improves both the strength and durability of concrete, both of which can be leveraged to make more sustainable concrete, therefore increasing the likelihood of greater use of superplasticizers in order to advance sustainability.
A comprehensive understanding of the mechanism and structure-property relationship can enable better design of superplasticizers, especially polycarboxylate ether (PCE) materials. PCEs have wide variation on a common comb-branch polymer architecture. The variations can come from the length of the backbone chain or the type of backbone (acrylic vs methyacrylic). In addition, the side chains or teeth can vary, by composition (PEO/PPO), length, and number of side chains on each main chain. An anionically-charged backbone drives adsorption to the cationic cement surface, while sterically-stabilizing polyether teeth keep the cement particles from colliding and agglomerating. Backbone length and degree of substitution, as well as length of side chains, can all be varied, yielding a possible wide variation of performance in a number of areas including initial rate of workability gain, maximum achieved, retention of that gain, compatibility with alkalis, and others. A great deal of progress in this area has been made and thoroughly summarized in recent years [32,33]. A well-tested and robust tool for PCE selection can drive the industry towards ever more efficient water reduction, and by extension, more sustainable concrete.
Elucidation of the mechanism of interaction of PCEs with hydrate species will also drive product development. This may be especially true in regards to a better understanding of the complex interaction between PCEs and availability of sulfate. Recent work has made it clear that both inadequate sulfate and excess sulfate can impact the performance of PCEs, and moreover, PCE addition and timing can impact the amount of sulfate needed to control cementitious systems. Additionally, the impact of aluminum from SCMs, likely to be used in greater amounts to drive sustainability, further points to the need to continue advancing this line of inquiry.
![Figure 8: Scheme from Plank & Hirsh [34]. Absorbed polymer on the growing surface of ettringite.](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_8.5b242d5336c58.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 8: Scheme from Plank & Hirsh [34]. Absorbed polymer on the growing surface of ettringite.
Figure 8: Scheme from Plank & Hirsh [34]. Absorbed polymer on the growing surface of ettringite.
Low Sulfate PCE Interactions:
Plank and Hirsh [34] and Dalas et al. [35,36] have established the absorption of PCE on ettringite as illustrated in Figure 8, but the impact on macro properties including rheology, structure, and strength of concrete are not yet fully understood.
The interaction of PCEs with aluminate phases yielding organoaluminates has been studied [37], and more specifically, researchers examined PCE interaction with sulfates [38]. The latter is depicted in the general scheme for cement hydration in Figure 9, which elegantly summarizes the potential reactions of C3A with water, various levels of sulfate, and PCEs. High sulfate leads to ettringite formation; moderate sulfate leads to monosulfate (AFm) formation, but very low sulfate leads to AFm with intercalated PCE, thereby reducing the effectiveness of PCE.
![Figure 9: Scheme for hydration, showing impact of sulfate content. From Plank et al. [38]](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_9.5b242d54a487d.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 9: Scheme for hydration, showing impact of sulfate content. From Plank et al. [38]
Figure 9: Scheme for hydration, showing impact of sulfate content. From Plank et al. [38]
Effect of Higher Sulfate in Competitive Adsorption:
The impact of sulfate on PCE dose efficiency was studied directly by Yamada et al. [39], demonstrating PCE efficiency is lowered by high levels of sulfate, which is attributed to competition between sulfate and the anionic backbone of PCEs.
PCEs can slow down the dissolution of the silicate phases [40] and probably impact the formation of etch pits [20]. The impact of PCE structure variation has been described by a sulfate sensitivity factor, which depends on the length of the ether chain and inversely on the square of the backbone length [32]. That is, for methacrylates, the polymer studied, the smaller the side chains and the longer the backbone, the less sensitive the PCE will be to sulfate. Expanding and validating this useful conclusion is a clear next step.
 Figure 10: Schematic diagrams of potential performance behavior of PCE’s as a function of availability of sulfate
Figure 10: Schematic diagrams of potential performance behavior of PCE’s as a function of availability of sulfate
It is clear that concrete mixtures can be both deficient in sulfate, and have too much sulfate for optimum PCE performance, indicating better mapping of sulfate content versus performance would be helpful. To date, research appears to have focused on either end of this spectrum. Ideally, performance diagrams encompassing both effects should be developed, resulting in data in the form illustrated in Figure 10. The interrelationship of molecular structure and cementitious mixture composition could then be more thoroughly understood and graphically represented to aid in finding the appropriate adjustment of sulfate levels.
Understanding competitive absorption becomes increasingly complex in combined admixture systems; behavior representative of current practice was reported by Bessaies-Bey et al. [41], who studied competition between PCEs and gluconates and hydroxyethyl cellulose ether. These studies are helpful conceptually – the challenge researchers face is integrating this work with all of the other specific studies, including those described above, to build a global view of the situation at the surface competition during hydration.
The use of very small reactive particles as space-filling, load-transferring materials is one of the key features of ultra-high performance concrete; such concretes are a promising strategy to reduce weight and cement use while maintaining performance. These particles have very small diameters, very high surface areas, and often distinct surface chemistries from cement, and thus form another kind of competitive adsorption. The interaction of PCEs in admixtures with one such material, silica fume, has been studied [42] using two particles (type I cement and silica fume) and four PCEs. The conclusion of this study was that different PCEs adsorbed best on each of the two particles, and that a blend of PCEs would make the best admixture for this system. More work along these lines is required as the demand for ultra-high strength concrete increases.
Finally, the use of supplementary cementitious materials is part of the path towards sustainability. Fly-ash (type F) is a common SCM with the well-known deficit of slow reaction and strength development, which is not always acceptable. Fly-ash reactivity can be accelerated by the use of alkali, but this may not be compatible with PCE-based superplasticizers. Marchon et al. [32] approached this problem from a competitive adsorption perspective, concluding that the decrease in workability is due to the competition between PCE and hydroxide ions for adsorption on the cement and fly-ash particle surfaces. A broader study, perhaps a next step, might involve non-carboxylate cement-binding functional groups, such as phosphates or phosphonates, which may compete more effectively with hydroxide.
This section described competitive behavior in normal systems. Later on, a real world example highlighting the dramatic influence of sulfate availability will be presented. This observation may be related to the sensitivity of ferrite phase reactivity to dispersion, a research area that will require further exploration in the future.
Retarders
Retarders describe a broad class of chemical admixtures whose primary function is to increase the time available for contractors to reliably transport, apply, and finish a given concrete system before the material hardens. More specifically, these compounds, sometimes referred to as delayed accelerators, act at the interface of the aluminate, silicate, and aluminosilicate phases of cement particles where they postpone the acceleration period that occurs during hydration without adversely affecting long-term strength development of the cementitious system. Until recently, the location and mechanism of their action has been unclear, but recent advances reviewed here provide greater clarity.
Although essential admixtures such as lignosulfonate and/or polycarboxylate-based water reducers may achieve the desired retardation effect independently, several classes of small molecules including carbohydrate derivatives (namely sugars), phosphonates, and metal oxides can be incorporated into admixture formulations to more effectively achieve tunable set retardation. From the aforementioned compounds, sugars are a very attractive class of chemicals that are readily available and relatively inexpensive. They have been studied for several decades, and numerous research groups have determined that performance is strongly dependent on chemical structure. Thomas and Birchall [43], for example, ranked several relevant sugar molecules based on retardation performance of OPC and determined the following:
Strong Retarders
sucroseb
raffinoseb
Moderate Retarders
glucose a
maltosea
lactosea
cellobiosea
Non-Retarders
α-methyl glucosideb
trehaloseb
At this stage, it is critical to point out that in addition to the differences in the chemical structure of the sugar molecules highlighted in the list above, due to the strongly basic (> pH 12) environment of hydrated cement systems, the stability of reducing sugars is very poor, thus a number of degradation products may be responsible for retardation (or lack thereof) [44]. Such findings provoke important questions: Why are only certain non-reducing sugars excellent retarders? Does the behavior of intact sugar molecules differ from smaller fragments/degradation products? Despite on-going discussion over the years, the specific mechanism of retardation of cementitious systems has yet to be realized. However, the field’s understanding has progressed significantly, thanks to several powerful advances in NMR techniques.
Since retarders perform their intended function at the interface of various phases within a given cement particle, their mechanism may be difficult to probe. Fortunately, through the use of 1D and 2D solid-state 29Si and 27Al NMR methods, Chmelka and collaborators [45–48] have successfully produced high-resolution spectra of both anhydrous and hydrated cements and cement components, to track critical compositional changes of silicate and aluminate species that occur during setting, and by applying 13C and 31P NMR spectroscopy, specific interactions between these phases and retarders have been captured.
![Figure 11: Schematic diagram from Smith et al. [46] Adsorption mechanism on C3A or aluminate hydrates of a) Glucose degradation products (non-selective adsorption) compared to b) sucrose (selective adsorption on anhydrous aluminate phases.](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_11.5b242d53764f5.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 11: Schematic diagram from Smith et al. [46] Adsorption mechanism on C3A or aluminate hydrates of a) Glucose degradation products (non-selective adsorption) compared to b) sucrose (selective adsorption on anhydrous aluminate phases.
Figure 11: Schematic diagram from Smith et al. [46] Adsorption mechanism on C3A or aluminate hydrates of a) Glucose degradation products (non-selective adsorption) compared to b) sucrose (selective adsorption on anhydrous aluminate phases.
Further investigation by Smith and colleagues [46] determined that sucrose also exhibits selective adsorption to hydrating silicate surfaces, more specifically, C3S, effectively delayed the onset of C-S-H production. Additionally, in the same study using solid-state 13C NMR measurements, the researchers confirmed that in high alkaline conditions: (1) glucose completely degrades to saccharinic acids and carboxylic acids, (2) maltodextrin degrades partially, and (3) sucrose remains intact, as predicted by Previte [44]. Studies described in Smith et al. [47] demonstrate that all the detectable 13C interactions occurring during the dormant period were occurring on unreacted, but hydroxylated Q0 silicate phases, and by implication, the retardation was due to either free energy changes on the surface (reducing reactivity) or blockage of water access. This conclusion led Sandgodkar et al. to apply Dynamic Nuclear Polarization (DNP) NMR [48]. In this technique, microwave radiation excites electron spin of a biradical dissolved in anhydrous solvent in which the hydrated and dried sample is dispersed. This spin is transferred to the nuclei within approximately 1 nm of the solvent, thus focusing the analysis on the surfaces only, and dramatically enhancing the signal to noise ratio. After this technique was applied, it was clear that the 13C interactions with sucrose occur on the anhydrous silicate, C-S-H, and CH. Therefore, it appears sucrose may have impacted both dissolution and nucleation/growth of both C-S-H and CH, accounting for its power as a retarder. Figure 12 depicts an improved understanding of this complexity.
![Figure 12: NMR data from Sangokar [48] that support the ability of sucrose to enhance both dissolution of C3S and nucleation/growth of hydration products](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_12.5b242d5588248.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 12: NMR data from Sangokar [48] that support the ability of sucrose to enhance both dissolution of C3S and nucleation/growth of hydration products
Figure 12: NMR data from Sangokar [48] that support the ability of sucrose to enhance both dissolution of C3S and nucleation/growth of hydration products
In addition to sucrose, phosphonic acid and phosphonates are well-known extraordinary retarders that have even been described as superretarders. Through the use of DNP surface-enhanced NMR techniques, Sangodkar et al. [48] determined that while both sucrose and phosphonic acid effectively inhibit the hydration of silicate phases, the respective adsorption behaviors observed strongly depend on their specific molecular structure. Sucrose preferentially adsorbs at silanol and silicate hydrates via hydrogen bonding. Conversely, phosphonates adsorb nonspecifically, principally by electrostatic interactions. Thanks to this new application of DNP-enhanced NMR spectroscopy to elucidate molecule-specific surface interactions, significant progress in the development of optimized retarders can be expected.
Moving forward, the collective work produced by the Chmelka group has vastly improved the overall understanding of retarders. In addition to distinguishing the specific cement phases involved and how their rates of hydration are impacted by retarders, the NMR techniques described in this section are expected to challenge researchers to focus on the wide range of possible adsorption behaviors (e.g. hydrogen bonding vs. electrostatic interactions) when designing more effective compounds that inhibit cement hydration. Their application is not limited to retarders; investigation of a broad range of sorbing molecules is theoretically possible. As the construction industry progresses towards more sustainable building materials, carefully tailored set retarding admixtures will provide concrete manufacturers with valuable mix design flexibility and to allow for adjustment at different temperature conditions.
Accelerators
Set and hardening accelerators have been used for more than a century to catalyze both the hydration of cement and activation of SCMs [49]. The most common type of accelerators used are soluble calcium or alkali salts of chlorides, bromides, nitrates, nitrites and thiocyanates, and alkanolamines such as triethanolamine (TEA), diethanol isopropanolamine (DEIPA), and triisopropanolamine (TIPA).
Accelerators catalyze the hydration reaction by shortening the induction period, a process that was suggested [5] to be controlled by the dissolution of C3S and the calcium concentration saturation level with respect to portlandite [5] .Using soft x-ray transmission microscopy, Juenger et al. [50] observed that the C-S-H products made with CaCl2 appear less dense in comparison to a control sample. The researchers proposed that CaCl2 increases flocculation of C-S-H to yield a more permeable product. This increased permeability enables water to more swiftly diffuse into the hydrating particle, while calcium and silicon ions diffuse away from the particle. More recent work made by Plusquellec and Nonat [51] confirmed Juenger’s findings. When changes in C-S-H suspensions with the addition of calcium chloride, bromide and nitrate were directly measured, it was observed that the addition of calcium salts increased the calcium concentration, leading to a modification of the surface properties of the resultant C-S-H. However, the chloride, bromide and nitrate anions were not adsorbed on the C-S-H particles. Both groups showed that new reaction products are not produced with the addition of CaCl2. Instead, C-S-H products with higher permeability were created to promote hydrate growth. These results indicate the presence of calcium cations may have a profound effect on both the ordering and morphology of the C-S-H.
Experimentally and via computational modeling, Nicoleau [52] determined a number of the key underlying mechanistic differences between various accelerators including nanosilica, fine portlandite, fine calcite, calcium chloride, sodium metasilicate pentahydrate, sodium hydroxide, calcium nitrate and sodium chloride. Based on the findings, hydration is influenced by (1) the number of C-S-H nuclei created (homogeneous or heterogeneous), (2) the dissolution rate of alite, (3) the growth of C-S-H in the parallel and perpendicular direction and (4) the permeability of the C-S-H. The researcher found that the main acceleration mechanism of the Ca-rich powders (portlandite and calcite) is via suppression of the induction time required to nucleate the initial C-S-H. The Si-rich powders (nanosilica and fumed silica) act as nucleation and growth promoters, even though the quantity of homogeneous C-S-H formed initially is less than that observed in the blank condition. Further, the addition of sodium metasilicate led to a huge precipitation of C-S-H nuclei. Nano-C-S-H particles acts by adding more nuclei in the pore solution instead of the alite grain surface and the perpendicular C-S-H growth is reduced. Ca salts also favor homogenous nucleation, but increase C-S-H perpendicular growth. Nicoleau also reported that a system with higher nuclei will create more permeable C-S-H, up to 40-50 nuclei positions, which is in concert with Juenger’s findings. This suggests that the kinetics of the hydration reaction are strongly influenced by the nucleation topology and the presence of divalent cations have profound effect on this.
When used at favorable dosage ranges that encourage acceleration of the hydration phases, alkanolamines provide early and/or late strength development. Typically, TEA gives early strength enhancement, TIPA gives late strength enhancement and DEIPA gives both early and late strength enhancement. These differences in performance are explained by the ability of the amines to remain in solution to effectively solubilize the iron phase [53]. More recently, researchers [54–56] determined that the improvement in strength was related to the difference in the morphology of the portlandite and the overall microstructure of resultant paste. Riding et al. [54] showed an acceleration of the hydration of the aluminate phases and Ma et al. [55] showed a promotion of the formation of the AFt and the acceleration of the transformation of AFt to AFm phases. Both studies found a change in the morphology of CH from hexagonal masses to thin plates with the addition of DEIPA, even at 0.02%. This change was even noticeable after only 10 hours of curing, indicating that this impact occurred at very early ages. Ma et al. observed DEIPA accelerated the hydration of alite at later stages. TGA and QXRD analysis also depicted a decrease in the portlandite content. A decrease in portlandite content was also reported by Ramachandra in C3S pastes treated with TEA [57].
![Figure 13: Schematic drawing from Rong et al. [56] showing the adsorption of TEA on CH surface](https://img.forconstructionpros.com/files/base/acbm/fcp/image/2018/06/figure_13.5b242d531bf0e.png?auto=format%2Ccompress&fit=max&q=70&w=400) Figure 13: Schematic drawing from Rong et al. [56] showing the adsorption of TEA on CH surface
Figure 13: Schematic drawing from Rong et al. [56] showing the adsorption of TEA on CH surface
Apart from hydration of the cement phases, Riding et al. observed that DEIPA also increased the hydration of the slag after 48 hours, possibly by either increasing the attack of the glass phases or by enhancing the mobility of elements dissolving from the slag (Figure 14). Berodier [31] clearly showed the filler effect enhancement of clinker hydration in presence of SCMs, but more research is needed to elucidate the exact mechanism of the SCM hydration enhancement and to give better guidance in determining the optimal dosage of additives to SCMs and cement.
Absorption of organic molecules on specific surfaces can serve to either retard or accelerate hydration. In the case of retardation, molecules block the dissolution of active sites. In the case of accelerators, inorganic calcium salts promote hydration by creating C-S-H nuclei in the pore solution, in addition to that on the alite surfaces, and alkanolamines are noted to adsorb on portlandite on the [001] growth plane, transforming the morphology of the portlandite to thin sheets or prisms, thereby allowing creation of finer microstructure. For many accelerators, it has been reported that the ultimate strength can be lower than the reference. There may be some parallels with Bentur’s findings on the calcium:silicate ratio’s influence on intrinsic strength of triclinic C3S-sulfate blends [23], where early strength was enhanced by greater reaction of the C3S through the addition of gypsum, while much of that benefit was lost at later ages due to lower intrinsic strength of the higher C:S ratio CSH. More work should be made in this area to understand the mechanism, so that robust solutions can be developed to overcome this weakness, and enable the placement of more sustainable concrete with less clinker and more SCMs.
Useful Tools to Enable Production of Sustainable Concrete with Improved Performance
Although the manufacture of concrete is generally a robust and forgiving process, variations in concrete performance are sometimes observed in the field, particularly in mixes made with high aluminate SCMs. As with most aluminate-silicate interactions, ensuring adequate sulfate appears to be key. Inadequate supply of sulfate can cause flash set, typically in situations of high aluminate, or long and unpredictable set, typically in situations of high ferrite.
In the previous section, we reviewed the underlying mechanisms of sulfate-aluminate-silicate interactions and how chemical admixtures impact these interactions. As different SCMs and admixtures are used in making concrete, it becomes difficult to predict the behaviors of the many different combinations. Thus, it is imperative to develop tools to quickly inform concrete manufacturers when incompatibility issues occur. In this section, we bring two real-life field issues to illustrate the use of calorimetry to help concrete manufacturers define approaches, such as the addition of more sulfate and the delayed addition of chemicals, to avoid deleterious hydration issues - flash set, set retardation, and slow strength development. In addition, we also describe the latest advance in the development of robust and responsive field in-transit concrete management systems to monitor and actively control the addition of admixtures during concrete delivery. This cutting-edge in-transit concrete management technology will provide important improvement in the making of sustainable composite systems with quality that call for high quantities and various classes of SCMs.
 Figure 15: Semi-adiabatic calorimetry of 20 % slag mixes from different cement truck loads dosed with a normal range of retarder. Temperature is relative to baseline.
Figure 15: Semi-adiabatic calorimetry of 20 % slag mixes from different cement truck loads dosed with a normal range of retarder. Temperature is relative to baseline.
Example 1: Use of sulfate addition to reduce extended retardation of slag mixes:
A real-life example of a long and unpredictable set is shown in Figure 15, where more than fifteen hours of difference in set time is seen from eight different truck load deliveries of cements when mixed with 20 % slag and a retarder dosed at normal range of ~200 ml/100 kg cementitious. Compositional analysis of the shortest and the two longest setting cements revealed that the SO3/C3A ratios were somewhat low at 0.23 - 0.26, but not unusual for this relatively high iron Type II cement. No other major compositional changes were noted from XRF and QXRD analysis to account for the large differences in set time.
 Figure 16 Laboratory isothermal calorimetry of (a) shortest setting cement load and (b) longest setting cement load. 80% OPC + 20% slag (green), 80% OPC + 20% slag + retarder (yellow), 80% OPC + 20% slag + retarder + 0.5 % SO3 as plaster (red) and 80% OPC + 20% slag + retarder + 1% SO3 as plaster (blue).
Figure 16 Laboratory isothermal calorimetry of (a) shortest setting cement load and (b) longest setting cement load. 80% OPC + 20% slag (green), 80% OPC + 20% slag + retarder (yellow), 80% OPC + 20% slag + retarder + 0.5 % SO3 as plaster (red) and 80% OPC + 20% slag + retarder + 1% SO3 as plaster (blue).
 Figure 16 Laboratory isothermal calorimetry of (a) shortest setting cement load and (b) longest setting cement load. 80% OPC + 20% slag (green), 80% OPC + 20% slag + retarder (yellow), 80% OPC + 20% slag + retarder + 0.5 % SO3 as plaster (red) and 80% OPC + 20% slag + retarder + 1% SO3 as plaster (blue).
Figure 16 Laboratory isothermal calorimetry of (a) shortest setting cement load and (b) longest setting cement load. 80% OPC + 20% slag (green), 80% OPC + 20% slag + retarder (yellow), 80% OPC + 20% slag + retarder + 0.5 % SO3 as plaster (red) and 80% OPC + 20% slag + retarder + 1% SO3 as plaster (blue).
Thus, both field and laboratory calorimetry were crucial in finding combinations with issues and in showing that addition of sulfate solved the problem despite no apparent large differences in time to sulfate depletion with the blank mixes. The mechanism whereby the extreme retardation occurred without added sulfate is not understood, and prediction of required increase in sulfate will require a better understanding of this phenomenon. Intercalation as described by Plank et al. [38] and others should have decreased retardation. Identification of retarder location by NMR as described by Sangodkar et al. [48], if practicable, could have provided insight.
Example 2: Addition delay of PCE superplasticizer reducing retardation:
Long retardation has also been found to be reduced by delay of addition of admixtures. In this example, an 85 % high-iron ASTM Type V cement + 15 % ASTM Class F fly ash mix failed to set for approximately 24 hours. This mix was made with three admixtures (a calcium nitrite based corrosion inhibitor, PCE superplasticizer, and a gluconate-based hydration-stabilizing admixture). Compositional analysis revealed this cement to have SO3/C3A ratio of 0.60, an amount typically considered adequate. However, less than half of SO3 was not present in a form of gypsum or plaster, suggesting that soluble sulfate may not be readily available for hydration. Particle size analysis by laser light scattering showed that the cement was high in ultra-fines, with approximately 10 % below 1 µm. These high ultra-fines would result in faster dissolution and are likely to require higher sulfate levels for development of proper hydration products.
 Figure 17: Isothermal calorimetry of admixture combination with added sulfate, compared to the non-admixtured reference mix. All made with 85% OPC and 15% fly ash. Reference without admixture (black), with admixtures but no added sulfate (red), 0.14% SO3 (light red), 0.50% (yellow), 0.75% (blue), 1.0% (green) added as plaster. To the left, 0-0.5 Hr., to the right 0.5-20 Hr.
Figure 17: Isothermal calorimetry of admixture combination with added sulfate, compared to the non-admixtured reference mix. All made with 85% OPC and 15% fly ash. Reference without admixture (black), with admixtures but no added sulfate (red), 0.14% SO3 (light red), 0.50% (yellow), 0.75% (blue), 1.0% (green) added as plaster. To the left, 0-0.5 Hr., to the right 0.5-20 Hr.
Extensive isothermal calorimetry work showed that the low sulfate content and high levels of microfines made the cement more susceptible to retardation, and that additional sulfate accelerated the reaction (Figure 17). In the 0 - 0.5 hour graph the much larger initial exotherm with admixtures is readily seen; also evident are significant early peaks which get progressively later as extra sulfate is added. Although they appear small, note the scale – the departure from a normal heat release decay is on the order of 3 - 4 mW/g and thus significant. One can hypothesize these are sulfate depletion peaks, but there is no XRD evidence to confirm this.
 Figure 18: Isothermal calorimetry of addition modes of admixtures - Reference without admixture (green), mix water addition of all admixtures (yellow), and mix water addition of all admixtures with 1 minute delay in addition of PCE (red). (a) first peak 30 minutes, (b) second peak 0.5 - 20 hours.
Figure 18: Isothermal calorimetry of addition modes of admixtures - Reference without admixture (green), mix water addition of all admixtures (yellow), and mix water addition of all admixtures with 1 minute delay in addition of PCE (red). (a) first peak 30 minutes, (b) second peak 0.5 - 20 hours.
In work not shown, delaying the addition of the calcium nitrite-based corrosion inhibitor had no effect. However, reducing the dosage of the corrosion inhibitor by 36 % restored the set time performance. Furthermore, a one-minute delay in addition of PCE showed a restoration of the set performance (Figure 18). Note the significant reduction in the size of the early exotherm, through 0.5 hours, but an appearance of an exotherm at about 1 hour of ~ 3 mW/g. The improvement in set was confirmed by full-scale truck testing, where upfront addition gave 25 hours initial set, and the identical mix with delayed addition of PCE set in 3 hours.
It is likely that the extreme retardation was impacted by very early dispersion of PCE in combination with low soluble sulfate and high levels of microfines, which resulted in rapid depletion of the sulfate. It does not appear related either to intercalation as described by Plank et al. – as no reduction of dispersion as measured by slump of concrete was noted, nor is it in the direction of decreased performance at higher sulfate as described by Yamada et al. It was found that while the cement and fly ash combination without admixtures showed a sulfate depletion point just before the silicate peak – a condition known to be predictive of potential issues with high admixture doses – the admixture combination used appeared to cause the sulfate depletion to occur at about 15 minutes. This implies that the self-limiting behavior of the C4AF, where the iron hydroxides produced in early hydration limit later reaction, was completely overcome by the admixture combination. What is surprising was that the addition of sulfate to move the sulfate depletion point out delivered normal setting of concrete, even though the depletion peak still appeared much ahead of the silicate peak at 1.6 hours. Similarly, delaying the PCE also resulted in what appears clearly to be an aluminate reaction at about 1.5 hours, yet subsequent hydration was normal with the delay. The underlying interactions between the admixtures-cement and SCMs in this example is worth pursing, as these are the components that would be commonly used to make sustainable concrete.
 Figure 19: Example of relative PCE dosages need for target 200 mm slump at placement with standard upfront addition compared to in-transit delayed addition.
Figure 19: Example of relative PCE dosages need for target 200 mm slump at placement with standard upfront addition compared to in-transit delayed addition.
Conclusions
As the industry moves forward to improve sustainability by use of more SCMs and higher admixture levels to drive down clinker content, challenging situations are expected to occur more frequently. A better understanding of these interactions via research and the development and implementation of field tools to help cement and concrete manufacturers improve production of high quality cement and concrete is critical. Fortunately, the industry is considerably more equipped to cope with these challenges today than in years past:
- More is known about the need to keep sulfate levels in cement from getting too low, and great advances over the last decade in laboratory techniques have helped users identify problematic combinations and demonstrate changes needed to resolve the issues.
- Recent developments in laboratory and field calorimetric techniques allow real mixes to be tracked and adjusted with simple changes in materials, time of addition, or changes in admixtures, prior to production. These adjustments can include addition of sulfates as illustrated above, replacement of materials (cement or SCMs), or a delay in addition of admixtures.
- In-transit concrete monitoring and management technology now exists to dose admixtures at any time from initial mixing until just before placement, and to make adjustments as needed during production.
On the research front, the following challenges need further investigation:
- Silicate dissolution by different admixtures made under realistic solution –to-solid and admixture-to-solid ratios.
- Role of aluminate adsorption with other species at C3S surface and impact of admixtures on these effects.
- Effect of sulfate on the dissolution of silicate phases and on the nucleation and growth of C-S-H and other hydration products.
- Impact of PCE architecture with various cementitious compositions, including use of high SCM levels with different sulfate levels.
- Competitive adsorption of different admixture species with the complex cementitious compositions.
- Impact in high ferrite cements.
- Activation by admixtures on SCM.
In summary, researchers should consider studying:
- More realistic systems: Fundamental research contributes to progress in cement and concrete technology but the field is faced with multi component systems (ternary cementitious system, several admixtures) which do not behave as simplistic systems used in research.
- The impact of sulfate to improve strength by controlling C3A reaction. Recent research had reported more effects related to sulfate including effect on C3S, C-S-H, CH, and also interaction with admixtures. Further investigation to provide an improved understanding and prediction of the required sulfate balance is important to produce sustainable concrete with higher use of SCMs and admixtures.
Ed. Note:The authors would like to thank David Myers, Nathan Tregger and Mark Roberts for their insightful suggestions.
References
[1] K.L. Scrivener, V.M. John, E.M. Gartner, Eco-Efficient cements: Portantial, economically viable solutions for low-CO2 cement-based materials industry, Paris, 2016.
[2] J. Cheung, Admixtures and Sustainability, Cem. Concr. Res. submitted (2016).
[3] J. Cheung, A. Jeknavorian, L. Roberts, D. Silva, Impact of admixtures on the hydration kinetics of Portland cement, Cem. Concr. Res. 41 (2011) 1289–1309. doi:10.1016/j.cemconres.2011.03.005.
[4] K.L. Scrivener, P. Juilland, P.J.M. Monteiro, Advances in understanding hydration of Portland cement, Cem. Concr. Res. 78 (2015) 38–56. doi:10.1016/j.cemconres.2015.05.025.
[5] P. Juilland, E. Gallucci, R. Flatt, K. Scrivener, Dissolution theory applied to the induction period in alite hydration, Cem. Concr. Res. 40 (2010) 831–844. doi:10.1016/j.cemconres.2010.01.012.
[6] E.M. Gartner, J.F. Young, D.A. Damidot, I. Jawed, Hydration of Portland Cement, in: Struct. Perform. Cem., 2002: pp. 978–0.
[7] L. Nicoleau, A. Nonat, D. Perrey, The di- and tricalcium silicate dissolutions, Cem. Concr. Res. 47 (2013) 14–30. doi:10.1016/j.cemconres.2013.01.017.
[8] P. Juilland, E. Gallucci, Morpho-topological investigation of the mechanisms and kinetic regimes of alite dissolution, Cem. Concr. Res. 76 (2015) 180–191. doi:10.1016/j.cemconres.2015.06.001.
[9] L. Nicoleau, M.A. Bertolim, Analytical Model for the Alite ( C 3 S ) Dissolution Topography, J. Am. Ceram. Soc. 14 (2015) 1–14. doi:10.1111/jace.13647.
[10] A. Bazzoni, M. Cantoni, K.L. Scrivener, Impact of Annealing on the Early Hydration of Tricalcium Silicate, J. Am. Ceram. Soc. 97 (2014) 584–591. doi:10.1111/jace.12691.
[11] Q. Hu, M. Aboustait, T. Kim, M.T. Ley, J.W. Bullard, G. Scherer, J.C. Hanan, V. Rose, R. Winarski, J. Gelb, Direct measurements of 3d structure , chemistry and mass density during the induction period of C3S hydration, Cem. Concr. Res. 89 (2016) 14–26. doi:10.1016/j.cemconres.2016.07.008.
[12] E. Pustovgar, R.P. Sangodkar, A.S. Andreev, M. Palacios, B.F. Chmelka, R.J. Flatt, J.E. De Lacaillerie, Understanding silicate hydration from quantitative analyses of hydrating tricalcium silicates, Nat. Commun. (2016) 1–9. doi:10.1038/ncomms10952.
[13] J.J. Thomas, The Instantaneous Apparent Activation Energy of Cement Hydration Measured Using a Novel Calorimetry-Based Method, 3296 (2012) 3291–3296. doi:10.1111/j.1551-2916.2012.05396.x.
[14] L. Nicoleau, A. Nonat, A new view on the kinetics of tricalcium silicate hydration, Cem. Concr. Res. 86 (2016) 1–11. doi:10.1016/j.cemconres.2016.04.009.
[15] A. Bazzoni, Study of early hydration mechanisms of cement by means of electron microscopy, EPFL, 2014.
[16] E. Masoero, J.J. Thomas, H.M. Jennings, A Reaction Zone Hypothesis for the Effects of Particle Size and Water-to- Cement Ratio on the Early Hydration Kinetics of C 3 S, J. Am. Ceram. Soc. 975 (2014). doi:10.1111/jace.12713.
[17] G.W. Scherer, J. Zhang, J.J. Thomas, Nucleation and growth models for hydration of cement, Cem. Concr. Res. 42 (2012) 982–993. doi:10.1016/j.cemconres.2012.03.019.
[18] L. Nicoleau, E. Schreiner, A. Nonat, Ion-specific effects influencing the dissolution of tricalcium silicate, Cem. Concr. Res. 59 (2014) 118–138. doi:10.1016/j.cemconres.2014.02.006.
[19] F. Begarin, S. Garrault, F. Begarin, S. Garrault, Hydration of alite containing alumimium, in: Cem. Concr. Sci. Congr., 2009: pp. 9–12.
[20] P. Suraneni, R.J. Flatt, Use of micro-reactors to obtain new insights into the factors in fl uencing tricalcium silicate dissolution, Cem. Concr. Res. 78 (2015) 208–215. doi:10.1016/j.cemconres.2015.07.011.
[21] T. Chappex, K.L. Scrivener, The effect of aluminum in solution on the dissolution of amorphous silica and its relation to cementitious systems, J. Am. Ceram. Soc. 96 (2013) 592–597. doi:10.1111/jace.12098.
[22] A. Quennoz, K.L. Scrivener, Interactions between alite and C3A-gypsum hydrations in model cements, Cem. Concr. Res. 44 (2013) 46–54. doi:10.1016/j.cemconres.2012.10.018.
[23] A. Bentur, Effect of Gypsum on the Hydration and Strength of C3S Pastes, J. Am. Ceram. Soc. 59 (1976) 210–213. doi:10.1111/j.1151-2916.1976.tb10935.x.
[24] S. Galmarini, A. Aimable, N. Ruffray, P. Bowen, Changes in portlandite morphology with solvent composition: Atomistic simulations and experiment, Cem. Concr. Res. 41 (2011) 1330–1338. doi:10.1016/j.cemconres.2011.04.009.
[25] B. Mota, T. Matschei, K. Scrivener, The influence of sodium salts and gypsum on alite hydration, Cem. Concr. Res. 75 (2015) 53–65. doi:10.1016/j.cemconres.2015.04.015.
[26] E. Berodier, Impact of the Supplementary Cementitious Materials on the kinetics and microstructural development of cement hydration, EPFL, 2015.
[27] A. Quennoz, K.L. Scrivener, Hydration of C3A–gypsum systems, Cem. Concr. Res. 42 (2012) 1032–1041. doi:10.1016/j.cemconres.2012.04.005.
[28] W. Lerch, “ The Influence of Gypsum on the Hydration and Properties of Portland Cement Pastes ,” Proc. Am. Soc. Test. Mater. 46 (1946) 1–48.
[29] M. Antoni, J. Rossen, F. Martirena, K. Scrivener, Cement substitution by a combination of metakaolin and limestone, Cem. Concr. Res. 42 (2012) 1579–1589. doi:10.1016/j.cemconres.2012.09.006.
[30] V. Kocaba, Development and Evaluation of Methods to Follow Microstructural Development of Cementitious Systems Including Slags, 4523 (2009).
[31] E. Berodier, K. Scrivener, Understanding the Filler Effect on the Nucleation and Growth of C-S-H, J. Am. Ceram. Soc. 10 (2014) 1–10. doi:10.1111/jace.13177.
[32] D. Marchon, U. Sulser, A. Eberhardt, R.J. Flatt, Molecular design of comb-shaped polycarboxylate dispersants for environmentally friendly concrete, Soft Matter. 9 (2013) 10719–10728. doi:10.1039/c3sm51030a.
[33] D. Marchon, S. Mantellato, A.B. Eberhardt, R.J. Flatt, Adsorption of chemical admixtures, in: Sci. Technol. Concr. Admixtures, 2016: pp. 219–256.
[34] J. Plank, C. Hirsch, Impact of zeta potential of early cement hydration phases on superplasticizer adsorption, Cem. Concr. 37 (2007) 537–542. doi:10.1016/j.cemconres.2007.01.007.
[35] F. Dalas, A. Nonat, S. Pourchet, M. Mosquet, D. Rinaldi, S. Sabio, Tailoring the anionic function and the side chains of comb-like superplasticizers to improve their adsorption, Cem. Concr. Res. 67 (2015) 21–30. doi:10.1016/j.cemconres.2014.07.024.
[36] F. Dalas, S. Pourchet, D. Rinaldi, A. Nonat, S. Sabio, M. Mosquet, Modification of the rate of formation and surface area of ettringite by polycarboxylate ether superplasticizers during early C3A-CaSO4 hydration, Cem. Concr. Res. 69 (2015) 105–113. doi:10.1016/j.cemconres.2014.12.007.
[37] C. Giraudeau, J.B. d’Espinose de Lacaillerie, S. Zied, N. Andre, F. Robert, Surface and Intercalation Chemistry of Polycarboxylate Copolymers in Cementitious Systems, J. Am. Ceram. Soc. 92 (2009) 2471–2488. doi:10.1111/j.1551-2916.2009.03413.x.
[38] J. Plank, D. Zhimin, H. Keller, F. Hössle, W. Seidl, Fundamental mechanisms for polycarboxylate intercalation into C3A hydrate phases and the role of sulfate present in cement, Cem. Concr. Res. 40 (2010) 45–57. doi:10.1016/j.cemconres.2009.08.013.
[39] K. Yamada, S. Ogawa, S. Hanehara, Controlling of the adsorption and dispersing force of polycarboxylate-type superplasticizer by sulfate ion concentration in aqueous phase, 31 (2001) 2–4.
[40] D. Marchon, Controlling Cement Hydration Through The Molecular Structure Of Comb Copolymer Superplasticizers, ETHZ, 2016.
[41] H. Bessaies-bey, R. Baumann, M. Schmitz, M. Radler, N. Roussel, Organic admixtures and cement particles : Competitive adsorption and its macroscopic rheological consequences, Cem. Concr. Res. 80 (2016) 1–9. doi:10.1016/j.cemconres.2015.10.010.
[42] C. Schröfl, M. Gruber, J. Plank, Preferential adsorption of polycarboxylate superplasticizers on cement and silica fume in ultra-high performance concrete ( UHPC ), Cem. Concr. Res. 42 (2012) 1401–1408. doi:10.1016/j.cemconres.2012.08.013.
[43] J. Thomas, L Birchall, The retardation action of sugars on cement hydration, Cem. Concr. Res. 13 (1983) 830–842.
[44] R. Previte, Some insights on the mechanism of saccharide set retardation of portland cement, Cem. Concr. Res. 1 (1971) 301–316. doi:http://dx.doi.org/10.1016/0008-8846(71)90005-6.
[45] A. Rawal, B.J. Smith, G.L. Athens, C.L. Edwards, L. Roberts, V. Gupta, B.F. Chmelka, Molecular Silicate and Aluminate Species in Anhydrous and Hydrated Cements, J. Am. Ceram. Soc. (2009) 161–167.
[46] B.J. Smith, A. Rawal, G.P. Funkhouser, L.R. Roberts, V. Gupta, Origins of saccharide-dependent hydration at aluminate , silicate , and aluminosilicate surfaces, in: Proc. Natl. Acad. Sci., 2011: p. 8949:8954. doi:10.1073/pnas.1104526108.
[47] B.J. Smith, L.R. Roberts, G.P. Funkhouser, V. Gupta, B.F. Chmelka, Reactions and Surface Interactions of Saccharides in Cement Slurries, Langmuir. (2012). doi:dx.doi.org/10.1021/la3015157.
[48] R.P. Sangodkar, B.J. Smith, D. Gajan, A.J. Rossini, L.R. Roberts, G.P. Funkhouser, A. Lesage, L. Emsley, B.F. Chmelka, H. Champs, I. De Sciences, A. Cnrs, E.N.S. Lyon, U.C.B. Lyon, U. De Lyon, Influences of Dilute Organic Adsorbates on the Hydration of Low- Surface-Area Silicates, J. Am. Ceram. Soc. 137 (2015) 8096–8112. doi:10.1021/jacs.5b00622.
[49] H.C. Vollmer, Calcium Chloride in Concrete, in: Bibliogr. 13, Highw. Res. Board, 1952: p. 58.
[50] M.C.G. Juenger, P.J.M. Monteiro, E.M. Gartner, G.P. Denbeaux, A soft X-ray microscope investigation into the effects of calcium chloride on tricalcium silicate hydration, Cem. Concr. Res. 35 (2005) 19–25. doi:10.1016/j.cemconres.2004.05.016.
[51] G. Plusquellec, A. Nonat, Interactions between calcium silicate hydrate ( C-S-H ) and calcium chloride , bromide and nitrate, Cem. Concr. Res. 90 (2016) 89–96. doi:10.1016/j.cemconres.2016.08.002.
[52]L. Nicoleau, Accelerated growth of calcium silicate hydrates : Experiments and simulations, Cem. Concr. Res. (2011) 1–10. doi:10.1016/j.cemconres.2011.04.012.
[53] E. Gartner, D. Myers, Influence of tertiary alkanolamines on Portland cement hydration, J. Am. Ceram. Soc. 76 (1993) 1521–1530. doi:10.1111/j.1151-2916.1993.tb03934.x.
[54] K. Riding, D. a. Silva, K. Scrivener, Early age strength enhancement of blended cement systems by CaCl2 and diethanol-isopropanolamine, Cem. Concr. Res. 40 (2010) 935–946. doi:10.1016/j.cemconres.2010.01.008.
[55] S. Ma, W. Li, S. Zhang, Y. Hu, X. Shen, Study on the hydration and microstructure of Portland cement containing diethanol-isopropanolamine, Cem. Concr. Res. 67 (2015) 122–130. doi:10.1016/j.cemconres.2014.09.002.
[56] Y.-R. Zhang, X. Kong, Z.-C. Lu, Z.-B. Lu, Q. Zhang, B. Dong, F. Xing, Influence of triethanolamine on the hydration product of portlandite in cement paste and the mechanism, Cem. Concr. Res. 87 (2016) 64–76. doi:10.1016/j.cemconres.2016.05.009.
[57] V.S. Ramachandran, Influence of Triethanolamine on the Hydration Characteristics of Tricalcium Silicate, J. Appl. Chem. Biotechnol. 22 (1972).
[58] ASTM C989 / C989M-16e1, Standard Specification for Slag Cement for Use in Concrete and Mortars, ASTM International, West Conshohocken, PA, 2016, www.astm.org
[59] N. Tregger, Automated slump management technology for consistent quality concrete, in: Transit Qual. Control. ACI 238 Work. Fresh Concr. - ACI Conv. Spring, 2015.
[60] K.H. Khayat, N.A. Libre, Automated Measurement and Control of Concrete Properties in a Ready Mix Truck with VERIFI, 2014.





























