Article originally published on Noria.com.
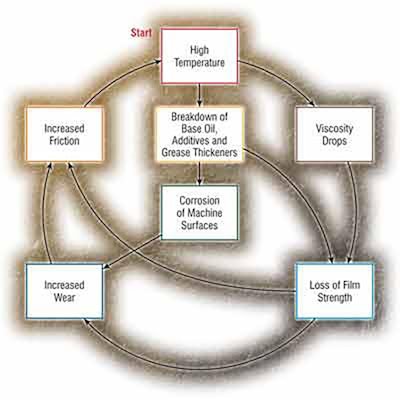
Lubricants, once they've exceeded their base activation temperature, will degrade (oxidize) twice as fast for every 18°F increase in temperature.
With oils, the chemical reaction that typically causes base oil degradation and additive depletion is oxidation. The activation energy required to induce oxidation in oil is high compared to other chemical reactions. The presence of contaminants such as water and certain metal particles in the oil can considerably speed up the process, thus increasing the activation rate. For most in-service mineral oils with typical contaminants, the activation energy for oxidation corresponds to a doubling for every 18°F temperature increment.
(read the entire article, "Advice for Extending Lubricant Life", at Noria.com...)


















